Training package on institutional AMR strategy development
Building an institutional AMR strategy
in order to reduce the spread of AMR and reduce operational costs
with rapid microbiological tests based on
the fastest possible detection of the most relevant and most common antimicrobial resistance mechanisms
Table of contents
I.1. Building up a knowledge pool on AMR and multiresistant bacteria
I.2. Assessing the local AMR situation in the hospital
I.3. Possibilities and advantages of the rapid AMR test and how to measure its impact
I.4. Planning the laboratory process with rapid AMR diagnostic test
I.5. Preparation of a meeting with the key stakeholders of the hospital
I. Preparatory phase
I.1. Building up a knowledge pool on AMR and multiresistant bacteria
WHO competency framework for health workers’ education and training on antimicrobial resistance:
https://apps.who.int/iris/bitstream/handle/10665/272766/WHO-HIS-HWF-AMR-2018.1-eng.pdf
This competency framework is intended to serve as a reference (for academic institutions, educators, accreditation bodies, regulatory agencies and other users) to help ensure that pre-service education and in-service training equip health workers with the requisite competencies to address AMR. It complements other relevant existing WHO guidance on AMR and lays the foundation for the development of more in-depth educational resources and AMR curricula.
Freely available courses about AMR:
https://www.ecdc.europa.eu/en/all-topics-z/antimicrobial-resistance/directory-guidance-prevention-and-control/training-courses – collection page with courses from
- several agencies: WHO, ECDC, USAID
- training institutes of some countries: Denmark, Sweden, UK
Antimicrobial resistance and stewardship e-Learning Repository of British Society from Antimicrobial Chemotherapy
https://bsac-jac-amr.com/jac-amr-resources/
I.2. Assessing the local AMR situation in the hospital
I.2.1. Identification of hospital departments with a high risk of infection/colonization with multiresistant bacteria
Start with existing information:
- Check the laboratory register for departments’ requests for microbiological testing.
- Identify the departments with a high rate of multidrug-resistant bacteria detected.
Suggested literature on risk assessment:
https://www.who.int/publications/i/item/9789241550178
The following hospital departments have a high risk of infection with multiresistant bacteria:
-
- Emergency Department
- Surgery Department
- Combustion Surgery Department
- Intensive Care Unit
- Oncology
I.2.2. Identification of patients with a high risk of infection/colonization with multiresistant bacteria
Individual-level risk assessment focuses on these factors
- health status of the patient,
- patient’s underlying diseases (internal risk factors)
- factors related to the care itself (external risk factors, e.g. device usage)
Suggested literature:
https://apps.who.int/iris/handle/10665/259462
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6055232/
https://pubmed.ncbi.nlm.nih.gov/34117191/
https://www.sciencedirect.com/science/article/pii/S1876034119302928
https://ccforum.biomedcentral.com/articles/10.1186/cc8877
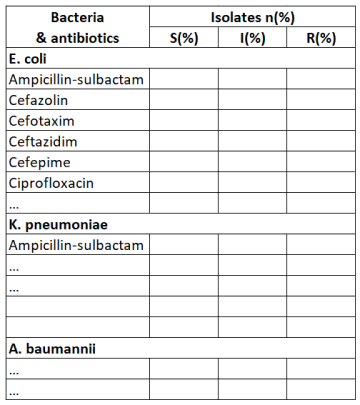
I.2.3. Monitoring antibiotic susceptibility rates for key bacteria and sharing reports with prescribers
Antimicrobial susceptibility testing with phenotypic methods is based on the measurement of the MIC (mg/L) and breakpoints to categorize bacteria and fungi as susceptible, intermediate or resistant.
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) has harmonized antibiotic interpretive breakpoints throughout Europe. They have been or are now being implemented in most countries in Europe and beyond.
Please consult the EUCAST website for information on performing and interpreting antimicrobial susceptibility testing
https://www.eucast.org/
https://pubmed.ncbi.nlm.nih.gov/26089441/
Knowledge of the likely pathogens and the likely susceptibility of these pathogens to antibiotics is essential to clinical decisions regarding empiric antibiotic therapy.
An example of a template for monitoring antibiotic susceptibility
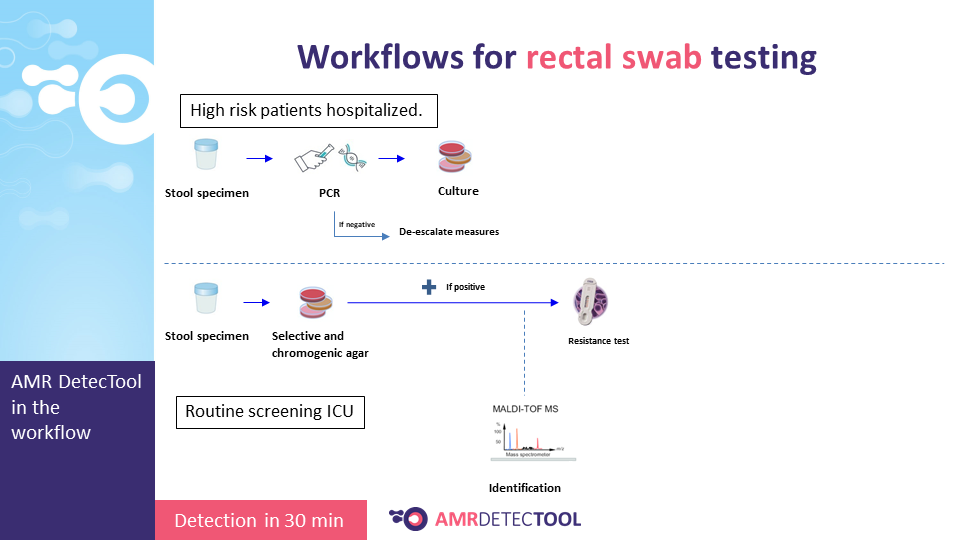
I.3. Possibilities and advantages of the rapid AMR test and how to measure its impact
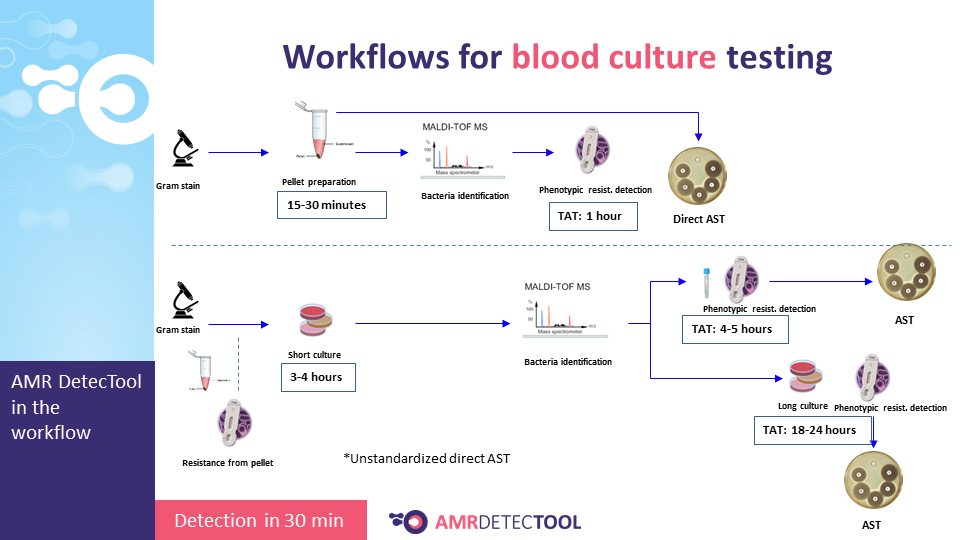
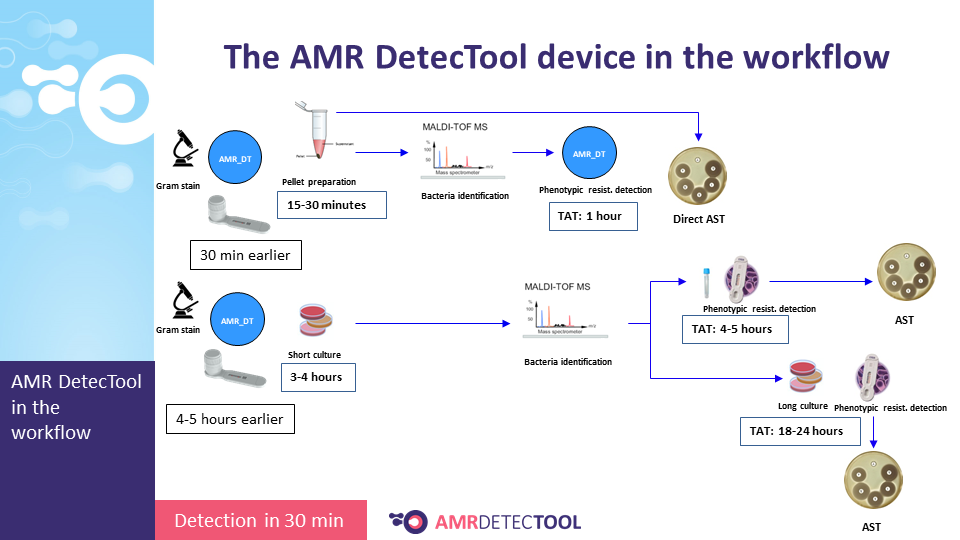
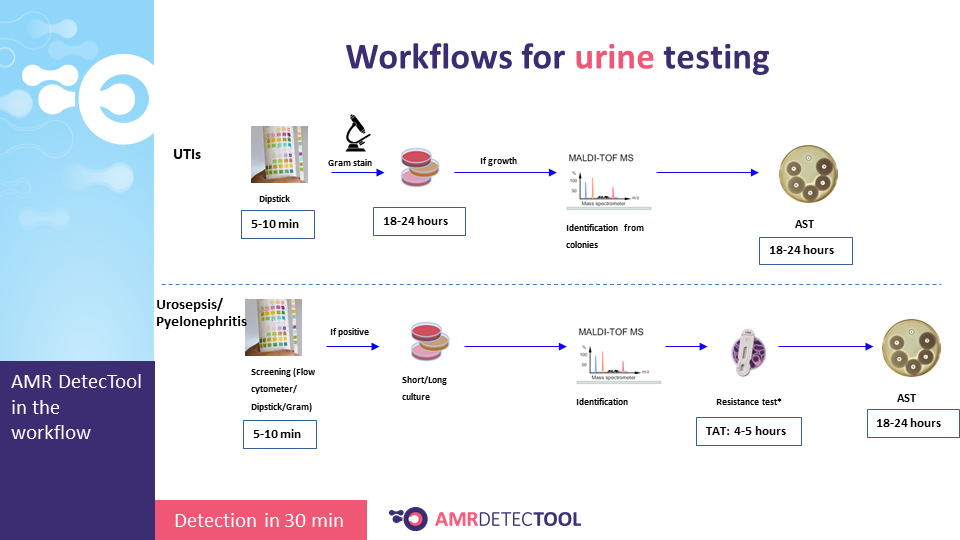
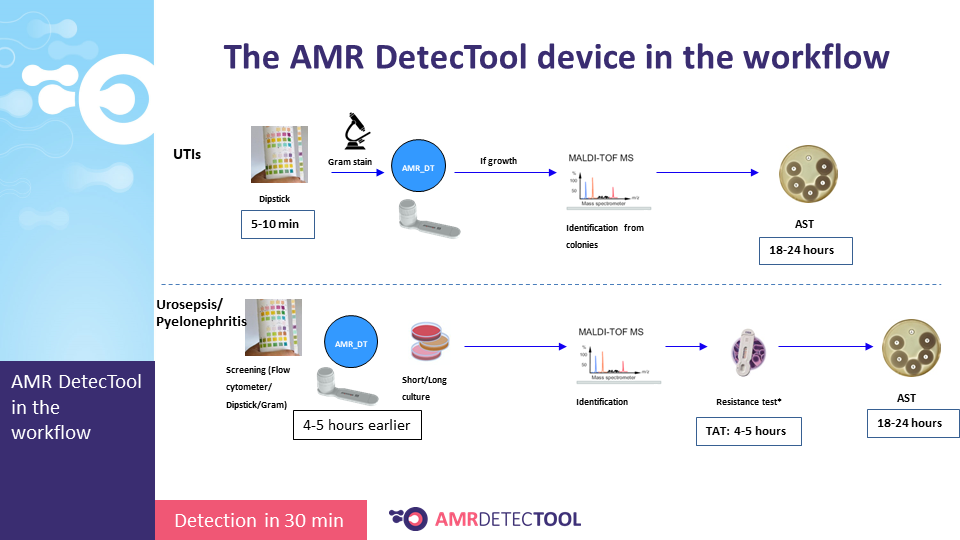
The AMR DetecTool device can detect bacteria’s most common resistance mechanisms directly from the patient’s sample (blood culture, urine, rectal swab, bronchoalveolar lavage / tracheal aspirate), shortening the workflow for the detection of multidrug-resistant bacteria to 30 minutes. Rapid detection is essential to prevent further spread and fight efficiently against MDR bacteria.
The main advantages of the AMR DetecTool include:
- quick and accurate treatments
- higher recovery rate of the patients
- decrease in mortality
- decrease in treatment time
- shorter hospital stays
- decreasing operational expenses, like the cost of treatment and hospital day
- reducing the spread of AMR – by rapidly detecting both colonisation and infection
Resistance mechanisms detected:
- ESBL: CTX-M MULTI
- Carbapenemases: KPC, OXA, VIM, IMP, NDM
- VAN A/B: vancomycin-resistant Enterococci
- OXA-Ab: OXA-type carbapenemases in Acinetobacter sp.
- 3GC: third-generation cephalosporin resistance
The Carba5 test detects OXA, KPC, VIM, IMP and NDM enzyme types; thus, specific antibiotics can be prescribed. OXA & KPC can be treated with meropenem + beta-lactamase inhibitor.
Measuring the cost savings
Cost calculation is based on the below cost items related to laboratory AMR testing:
- cost of sampling
- cost of patient admission and hospital day
- staff costs
The cost savings are calculated based on whether the empiric treatment needs to be changed based on the results of the rapid diagnostic tool and how much faster the results are obtained with the rapid test than with traditional methods.
Measuring the reduction of the spread of AMR requires monitoring healthcare-associated infections: recording, analyzing and reporting information about the patient, the site involved, the associated organism, risk factors and the proportion of infections caused by multidrug-resistant bacteria.
Information and support:
The Healthcare-Associated Infections Surveillance Network (HAI-Net)
https://www.ecdc.europa.eu/en/about-us/networks/disease-networks-and-laboratory-networks/hai-net-about
Electronically assisted surveillance systems of healthcare-associated infections: a systematic review
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6976884/
I.4. Planning the laboratory process with rapid AMR diagnostic test
I.4.1. Specimen selection and collection
The AMR DetecTool rapid tests need the same specimen as the currently used laboratory tests: blood culture, urine, rectal swab, and no required extra sampling.
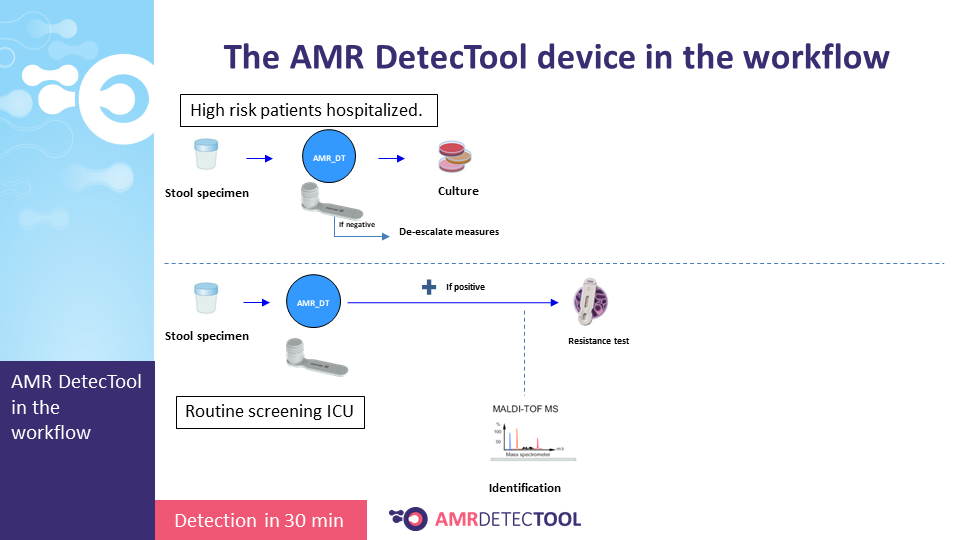
I.4.3. Integrating the device into the workflow
Integrate the AMRDetecTool after the gram stain, from the positive blood culture and after screening and microscopic test from the urine, before identification at the species level.
The workflow sections where the AMR DetecTool is to be integrated
I.5. Preparation of a meeting with the key stakeholders of the hospital
Suggested stakeholders for cooperation:
- hospital management
- finance unit
- microbiology department
- clinical prescribers, e.g. ID specialists, intensivists
- medical heads of departments, especially of departments with a high risk of infection/colonisation with multiresistant bacteria
- infection prevention and control practitioners
- quality-of-care/patient-safety teams
- antimicrobial stewardship team
Suggested topics for discussions:
- advantages and possibilities of rapid AMR testing with the AMRDetecTool
- measuring the impact of the implementation of the rapid testing processes
- types of rapid tests
- rapid, accurate treatment based on rapid testing results
- selecting patients for rapid testing (based on risk assessment at the institutional and personal level) – the issue of infection/colonisation
- reporting the results of the AMR DetecTool device
- feedback from the clinicians to the lab concerning the prescribed antibiotics (the issue of empirical treatment)
Additional background information to prepare for the meeting
1. The issue of empirical treatment
“Because microbiological results do not become available for 24 to 72 hours (with the currently used standard methods), initial therapy for infection is often empiric and guided by the clinical presentation. It has been shown that inadequate therapy for infections in critically ill, hospitalized patients is associated with poor outcomes, including greater morbidity and mortality as well as increased length of stay.
Therefore, a common approach is to use broad-spectrum antimicrobial agents as initial empiric therapy.
Hospital-acquired infections are frequently related to the presence of invasive devices and procedures that result in loss of the normal barriers to infection, as is the case with intravascular catheter-associated bacteremia, ventilator-associated pneumonia, and catheter-associated urinary tract infections (UTIs). They are commonly caused by drug-resistant organisms, which are often endemic in hospitals because of the selection pressure from antimicrobial use. In selecting empiric antimicrobial therapy for such infections, clinicians should consider the following:
- the site of infection and the organisms most likely to be colonizing that site (e.g., intravascular catheter-associated bacteremia is frequently a result of colonization and infection caused by staphylococci present on the skin)
- prior knowledge of bacteria known to colonize a given patient (e.g., a nasal screening swab [currently conducted routinely by many hospitals before admitting patients to the intensive care unit] may indicate that the patient is colonized with MRSA)
- the local bacterial resistance patterns or antibiograms available for important pathogens at most hospitals.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3031442/
General Principles of Antimicrobial Therapy
What is the evidence base of used aggregated antibiotic resistance percentages to change empirical antibiotic treatment? A scoping review:
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00700-X/pdf
2. Communication between the laboratory and the hospital departments
During a microbiological diagnostic process, some preliminary results are generated; the relevant ones that base a therapeutic decision need to be communicated to the clinicians to facilitate patient care.
***
Microbiological labs need to ensure expertise in interpreting results.
As laboratory testing becomes more specialized and faster, the need to accurately interpret test results is ever more important.
When is critical information communicated to hospital clinical staff for decision-making, such as selection or modification of an antibiotic regimen, who must be involved? In most cases, the decision is easily made by the primary service. However, late at night, especially when coverage physicians not familiar with the case are involved or the decision rests on incomplete pathogen identification or antibiotic susceptibility results, who does the work? Current approaches include programs to educate healthcare providers about new diagnostic tests, antibiotic options, and when to contact a specialist not currently involved in the case.
Communication Between Clinicians and the Hospital-Based Microbiology Laboratory: Strategies for 2018 and Beyond
https://ir.library.louisville.edu/cgi/viewcontent.cgi?article=1041&context=jri
I.6. Meeting the key stakeholders
Depending on the local AMR and HR conditions and the institutional history of fighting AMR, several meetings may be necessary. Try to involve as many departments as possible to join the first meeting.
In addition to discussing the topics suggested above under I.5., present existing relevant data/information at this meeting, including:
- antibiotic resistance data,
- empirical treatment protocols,
- preliminary cost calculations.
During the meeting, discussing cooperation and communication between the laboratory and clinical departments is essential.
Make a memo of the issues discussed, and decisions made, and provide feedback to participants.
II. Implementation phase
Implementation is the process that turns plans into actions – in order to accomplish goals.
Rapid AMR diagnostics may be integrated into the antimicrobial stewardship program (ASP). If there is an antimicrobial stewardship team in the hospital, they can help to establish the integration of a rapid diagnostic device into the diagnostic pathway. In addition, the Quality Improvement team can play an important role as they use process implementation science in daily practice.
Implementation science studies methods and strategies facilitating the uptake of interventions that have proven effective in regular use by hospital staff and management.
Literature on implementation science:
Website of the Institute for Healthcare Improvement (IHI)
https://www.ihi.org/resources/Pages/HowtoImprove/ScienceofImprovementImplementingChanges.aspx
Hospital-based interventions: a systematic review of staff-reported barriers and facilitators to implementation processes
https://implementationscience.biomedcentral.com/articles/10.1186/s13012-018-0726-9
The support of hospital management is vital in the efforts to transform the AMR detection process at the institutional level.
III. Continuous operational and monitoring phase
Continue the previous monitoring activities concerning AMR, and data on prescription and consumption of antibiotics. Keep a regular presentation system of these results to the hospital management.
Check which data can be monitored using extracts from existing IT systems and which require manual auditing. To further the development of monitoring, try to involve the IT department.
Literature on the further development of monitoring:
Advances in electronic surveillance for healthcare-associated infections in the 21st Century: a systematic review
https://www.journalofhospitalinfection.com/article/S0195-6701(13)00130-8/fulltext
Make the cost calculations and demonstrate the cost savings reached by the implementation of the rapid testing processes.
Keep high awareness about AMR, including its impact on patient safety.
We drew on the literature on starting and implementing antimicrobial stewardship programs to develop this guide.
https://www.sciencedirect.com/science/article/pii/S1198743X19304483

Copyright 2020 – AMRDETECTOOL